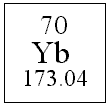
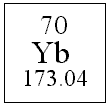
Marc Duyungan
The Ytterbium times
online news from distinguished sources regarding the element ytterbium
Today's Top Stories=Anagrams, Crystal Structure, Movie Reviews, Lab Safety
NEWS BRIEFS:
Scientists Find Ytterbium on Periodic Table: "...[Mendeleev] was...smart."
Through intense scrutiny of a so-called "periodic table" found in any chemistry textbook, a group of scientists has inferred a few key properties of the element Ytterbium. With an atomic number of 70, and an atomic mass of 173.04 g/mol, Ytterbium also has common oxidation states of 2+ and 3+. After examining the table further and realizing how little Mendeleev had to go on when he created said table, members of the group were quoted as saying, "Damn, that dude was way smart."
Student Makes Stunning Discovery
Amazed at his own skill at wordplay, a local high school student, bored in his chemistry class, discovered that the letters in Ytterbium could be rearranged to spell I'm Buttery. Experts were astounded. "We've dreamed of the anagram-atical possibilities of the periodic table for years now, but this...this is just amazing. This is light-years beyond the stuff we've been working with, like rearranging Hydrogen into No Hydreg. I mean, that doesn't even make sense! But this kid, this kid's put together something that might actually be mistaken for a sentence...I'm flabbergasted."
Ytterby Resident Proud of Heritage
Hans Yodeler, life-long resident of Ytterby, was proud to learn that Ytterbium was named after his village. "Yah, I am honored to learn such things," said Yodeler...other words of wisdom were also spoken, but this reporter did not feel the need to write them down. Ytterbium was apparently discovered by Jean de Marignac, in Switzerland around 1878 [Ed. note: Ytterbium is not found alone in nature; it is found in ore monazite sand and is separated from said compound]. Known primarily for their clocks, chocolate, and neutrality; Swiss everywhere felt their national pride swell upon hearing the news.
Although he has been dead for over 100 years, de Marignac was quoted as saying, "I am very proud of my Ytterbium, with its silvery white color, and solid state at 298 Kelvin. It is also soft, malleable, lustrous, and ductile, revealing itself as a metal."
College Student Pressured Into Examining Crystal Structure
Various reports from local sources paint a disturbing picture of recent campus events at Pomona College. GChem Student ('04) was, through severe cajoling and repeated utterances of "everybody's doing it," coerced by a group of anonymous friends into examining the 3-dimensional crystal structure of Ytterbium. After realizing the cubic-closest packed structure of the element, Student became bored and ingested various "substances." Upon returning to the structure while altered, Student said, "Whoa! I can, like, feel the colors, man!"
If you want to be able to play around with the crystal structure of Ytterbium, first visit http://www.mdlchime.com/chime/ to pick up a plug-in, then head on over to http://www.webelements.com/webelements/elements/text/Yb/xtal-pdb.html to check it out. (The Ytterbium Times in no way, shape, or form endorses the use of various substances to enhance the experience).
 Cubic Closest Packed Structure of Ytterbium
Cubic Closest Packed Structure of Ytterbium
Nerdy Lab Guys Find Ytterbium Isotopes; And There Was Much Rejoicing
Reports from Lab Man's laboratory in Austria have provided fascinating new insights into the world's supply of Ytterbium, determining that 32% of it is 174-Yb, 22% is 172-Yb, and 171-Yb, 173-Yb, 176-Yb each make up 13%. A random citizen, Joe Schmoe, upon being informed of this, was quoted as saying, "What? Ytterbium...Does that have anything to do with Yiddish? Maybe I Can't Believe It's Not Butter?" Clearly, this is an exciting time for all Ytterbium enthusiasts.
Film Reel: Review of "YtterbiumMan"
An intriguing tale of a rich, powerful yuppie (Tom Cruise), and how his life drastically changes when he meets his long-lost brother (Dustin Hoffman). Upon visiting Las Vegas, Hoffman's abilities turn out to be completely useless, seeing as card-counting is nowhere near his skills. Instead, he would jabber on about the physical properties, electronic configuration, and electronegativity of Ytterbium. "Melting point of 1097 K. Definitely 1097." Followed by his stirring deliveries of a boiling point of 1467 K, an electron configuration of [Xe] 6s2 4f14, and an electronegativity of 1.1, Hoffman's performance will definitely be noted by the Academy. *** on a **** scale.
Industry Experts Excited by Ytterbium's Uses
Industry insiders have hardly been able to contain their excitement upon learning of Ytterbium's (not-so) numerous uses. Apparently, one of the isopes can be used for portable X-rays, due to its radioactive nature. It can also be used for lasers, though this reporter's source was unspecific as to how this would be accomplished. It can also be used to improve the strength of stainless steel. While experts are excited, the general population seems to remain nonplussed.
Local Scientist Ignores Safety Precautions; Pays Price
Though he was warned repeatedly by lab instructors, GChem Student ('04) declined to wear his lab safety goggles during his weekly lab session. Upon being exposed to Ytterbium, Student quickly realized the reasons for the precautions. After getting it in his eyes and attempting to rub it out, he learned that it was both a skin and eye irritant. Professor Truttman then informed him of Ytterbium's highly toxic nature, and after doing some research, it is predicted that Ytterbium's teratogenic nature will give GChem Student an 80% probability of cancer.
Atomic Number=70
Atomic Mass=173.04 g/mol
Common Isotopes=174-Yb, 172-Yb, 176-Yb, 173-Yb, 171-Yb
Electron Configuration=[Xe] 6s2 4f14
Oxidation States=+2, +3
Physical Appearance=Silvery white, solid at 298 K
Physical Properties=Melting Point:1097K, Boiling Point:1467K, Cubic Closest Packed Structure
Electronegativity=1.1
Source=Found in ore monazite sand
Uses=Lasers, X-rays, improving stainless steel
Information used to build this site can be found at:
http://www.webelements.com/webelements/elements/text/Yb/key.html