Molybdenum
| Atomic Number: |
42 |
| Atomic Weight: | 95.94 g |
|
Melting
point: |
2896 K |
|
Boiling
point: |
4912 K |
|
Electronegativity |
|
|
Density: |
10.22 g/cc |
Crystal Structure: Cubic body centered
·
Electron Configuration:
1s2
2s2p6
3s2p6d10
4s2p6d5
5s1
·
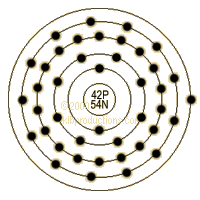
Electrons per Energy Level: 2,8,18,13,1
Shell Model:
· Discoverer: Carl Wilhelm Scheele
· Discovery Location: Sweden
· Discovery Year: 1778
· Name Origin: Greek: molubdos (lead).
· Sources: Found in the minerals molybdenite (MoS2)
and wulfenite (MoO4Pb).
· Uses: Used in steel, aircraft, missiles, filaments in electric heaters, lubricants and protective coatings in boiler plates.
|
Isotope |
Half
Life |
|
Mo-91 |
15.5
minutes |
|
Mo-92 |
Stable |
|
Mo-93 |
3500.0
years |
|
Mo-94 |
Stable |
|
Mo-95 |
Stable |
|
Mo-96 |
Stable |
|
Mo-97 |
Stable |
|
Mo-98 |
Stable |
|
Mo-99 |
2.74
days |
|
Mo-100 |
Stable |
|
Mo-101 |
14.6
minutes |
What is Molybdenum?
Molybdenum (Mo) is a refractory metallic element used principally as an alloying
agent in steel, cast iron, and superalloys to enhance hardenability, strength,
toughness, and wear and corrosion resistance. To achieve desired metallurgical
properties, molybdenum, primarily in the form of molybdic oxide or
ferromolybdenum, is frequently used in combination with or added to chromium,
columbium (niobium), manganese, nickel, tungsten, or other alloy metals.
Few of molybdenum's uses have acceptable substitutions.
Molybdenum
(often referred to as moly) is a metal that is gaining increasing significance
in our industrial world.
Where does Molybdenum come from?
Molybdenum is found the world over but only a few deposits warrant the extensive
mining,
milling
and processing facilities necessary for its economic recovery. By far
the largest part of world production comes from the Western Hemisphere with the
United States contributing the biggest share, Chile in the second place and
Canada in third. Outside of a modest output from various locations in China,
little molybdenum is mined in the rest of the world.
How is Molybdenum used?
After crushing, the molybdenum ore is concentrated by flotation to a product
consisting largely of molybdenite, which is molybdenite sulfide. It can be
purified to give a product used by lubricant manufacturers. Most of the
molybdenum concentrate is roasted to remove the sulfur and change the sulfide to
oxide. This oxide, which is known as technical
molybdic oxide, is the most common means of adding
molybdenum to steel as there is almost no loss of molybdenum during the usual
melting operations.
A mixture of technical molybdic oxide and iron oxide can be reduced to ferromolybdenum
by aluminium in a thermite reaction. Foundries generally use ferromolybdenum for
adding molybdenum to cast iron and steel, and steelmills may prefer it to the
technical molybdic oxide for some types of steels.
Pure
molybdic oxide is made by sublimation of technical oxide or
by calcining ammonium molybdate. Pure oxide is suitable for use by chemical and
catalyst manufacturers. Molybdenum metal powder is produced by hydrogen
reduction of the pure molybdic oxide or ammonium molybdate. In turn, this
molybdenum metal powder is consolidated into usable forms by vacuum melting or
by pressing and sintering.
Who uses Molybdenum?
Almost everyone uses molybdenum, even when they do not realize it. Superficially
molybdenum looks like many other gray metals, and this is one of the reasons
many do not appreciate the extent of its use. Everyone knows what copper looks
like and can immediately identify a cable as being copper. Not many can look at
a light bulb or a TV tube and tell which parts are molybdenum.
The picture becomes even more difficult in the case of the main use of
molybdenum as an alloying element added in small amounts to steels, irons and
nonferrous alloys. The increasing popularity of lubricants containing
Molysulfide has extended from heavy industry to service stations and home
workshops. Their black color and the inclusion of the term Moly in the lubricant
manufacturer’s trademark can identify these.
How much does it cost?
Molybdenum is sold on a spot basis in a variety of forms including molybdenum oxide, molybdate (a salt), and ferromolybdenum (an iron alloy containing up to 60 percent molybdenum). In late 1994 and early 1995 molybdenum prices reached a 15-year high of US$18.00/lb as a result of a large world steel demand; by the end of 1995 molybdenum prices had corrected to US$5.00/lb due to increased world production. China has accounted for between 85 and 90 percent of annual world ferromolybdenum exports since 1993, but with its own steel industry growing stronger, China will have less molybdenum to offer on the world market. Besides demand and China's lessened role, experts say the major influence on molybdenum prices will be whether primary producers - like Cyprus Amax Minerals, the world's largest producer, holding 35 percent of the global market - can modify their output to accommodate any shortfall between supply and demand.
What does molybdenum do?
The demands of industry are becoming constantly more severe. Engineers want
stronger, tougher materials with better hot strength, superior properties at low
temperatures, more corrosion resistance and added wear resistance so they can
design and build more efficient equipment to give us a better life. Molybdenum
helps meet these demands.
Just like other common alloying elements such as chromium and
nickel, molybdenum additions give alloy steel and iron a combination of
strength, toughness and wear resistance not possible with unalloyed steels. Its
extensive use is proof that, under many conditions, its inclusion (alone or with
other alloys) results in a more economical and serviceable part. Moreover,
molybdenum makes a unique contribution to hot strength, corrosion resistance and
toughness.
Increasing temperature raises the efficiency of most types of equipment from
steam turbines in central power stations to gas turbines in jet planes and
eventually automobiles. Relatively small molybdenum additions are in many cases
the best means of increasing hot strength. This applies not only to steel but
also to the nonferrous super-alloys with nickel or cobalt as a base. In some
aerospace and metalworking applications, molybdenum metal – either pure or
with small additions of other alloys – is needed as it stands up even at
temperatures where steel melts.
Because it keeps its strength and structure at high temperatures, molybdenum is
used largely in alloys
as a hardening agent. Steel-molybdenum
alloys are used to make high-speed cutting tools, as well as aircraft and
automobile parts. Thus, there is no substitute for molybdenum in its major
application as an alloying element.
Molybdenum
in sheet or wire form is used in X-ray tubes and electric furnaces. Molybdenum
has key nuclear-energy applications, and is found in critical rocket and missile
parts. It is also a catalyst in petroleum refining, filament material in
electrical applications, and an essential trace element in plant nutrition.
| Molybdenum was featured in an issue of the Flash comic strip, number 70, January 1993, page 6 | ||||||||||||||||||||
 |
||||||||||||||||||||
taken from: http://www.uky.edu/Projects/Chemcomics/html/molybdenum.html
What is molybdenum?
Molybdenum
is a silverish-white trace element with metallic properties. It is found most
often in soils and is usually absorbed through the consumption of plant
material. The human body contains about nine milligrams of molybdenum, with the
highest concentrations found in the liver, kidneys, bones and skin.
Why do we need it?
Molybdenum
is essential for the proper function of certain enzyme-dependent processes,
including the metabolism of iron. It also forms part of several body enzymes and
is needed to convert a substance called purine into uric acid. Molybdenum has
been used to treat copper toxicity in conditions such as Wilson's disease.
Preliminary evidence has suggested that molybdenum might prevent certain types
of asthma attacks. It has also been used with fluoride to treat dental decay.
How much molybdenum should
I take?
There is
currently no recommended daily allowance (RDA) for molybdenum. However, the
National Academy of Sciences has deemed the following amounts to be safe and
adequate in a normal diet:
What are some good sources of molybdenum?
Large amounts of molybdenum are found in milk, beans, dark green leafy vegetables, unrefined cereals and grains. Hard tap water can also supply molybdenum to the diet.
What can happen if I don't get enough molybdenum?
Molybdenum deficiency is virtually nonexistent in the U.S. and is usually seen only in people who have been on prolonged tube or intravenous feeding or have a genetic inability to metabolize molybdenum. Symptoms of deficiency include rapid heartbeat and breathing, headaches, night blindness, anemia, mental disturbances, nausea and vomiting. Some studies conducted in Japan and China have linked low levels of molybdenum with an increased risk of stomach and esophageal cancers.
What can happen if I take too much?
Molybdenum toxicity is extremely rare in the United States. Most health experts
agree that an intake of as much as 15 milligrams per day is safe; however, large
amounts can interfere with the absorption of copper. In rare cases, excessive
molybdenum consumption can cause nausea, diarrhea, or gout-like symptoms such as
joint pain and swelling.
What does it do?
Molybdenum is an essential trace mineral. It is needed for the proper
function of certain enzyme-dependent processes, including the metabolism of iron.
Preliminary evidence indicates that molybdenum, through its involvement in
detoxifying sulfites, might reduce the risk of sulfite-reactive asthma
attacks. However,
a physician should be involved in the evaluation and treatment of sulfite
sensitivity.
Where is it found?
The
amount of molybdenum in plant foods varies significantly and is dependent upon
the mineral content of the soil. The best sources of this mineral are beans,
dark green leafy vegetables,
and grains.
Hard tap water can also supply molybdenum to the diet.
Links:
International Molybdenum Association