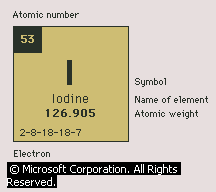
Iodine

![]()
The Basics
-Isotopes
|
Isotope |
Atomic mass (ma/u) |
Natural abundance (atom %) |
Nuclear spin (I) |
Magnetic moment (m/mN) |
|---|---|---|---|---|
|
127I |
126.904473 (5) |
100 |
5/2 |
2.81328 |
Iodine also has a useful radioactive isotope, iodine-131, which has a half-life of eight days. See the Uses section to find out more.
-Ground State Electronic Configuration
[Kr].4d10.5s2.5p5
-Oxidations States
-1 (iodides), +5 (iodates), and +7 (periodates).
-Physical Appearance
At room temperature, iodine is a crystalline solid that, when heated sublimes to a deep violet vapor that is irritating to the eyes, nose, and throat. See the Graphics section for pictures and a movie of it!
-Physical Properties
Iodine melts at 113.6° C (236° F) and boils at 185° C (365° F). It has an orthorhombic structure (see the Graphics section). The density at 293 K is 4.93 g/cm3.
-Source
Iodine is obtained from brines and from nitrate ores in which it exists as an impurity. Also, but not as much, iodine is derived from sea organisms, such as brown seaweeds, that concentrate iodine in their tissues.
-Price
$14.25 for 25 GM (= 57 cents per gram)
-Electronegativity
Pauling Electronegativity [/Pauling units]: 2.66
This site was created by Ryan
Scholl using Microsoft Front Page. You can E-mail me by clicking the image below
if you have comments, gripes, or suggestions. Last edited: 05/21/01
![]()