 Paige's Dysprosium
Page
Paige's Dysprosium
Page Paige's Dysprosium
Page
Paige's Dysprosium
Page
WHAT IS DYSPROSIUM?
An infectious disease... An exotic food... An ancient city...


No, actually, dysprosium (Dy) is the 66th element in the periodic table and the ninth rare earth metal in the lanthanide series.

The name dysprosium is derived from the Greek word "dysprositos," meaning hard to get at. In many ways this lesser known, somewhat mysterious element is true to its name. It is difficult to isolate and limited in its uses. Fortunately, this website will reveal the wonder of dysprosium, and answer any dysprosium related questions.

Facts about Dysprosium
History: Dysprosium was discovered in 1886 by French chemist Andre Lecoq de Boisbaudran. However, it was not isolated at this time. Neither the metal nor the oxide was available in pure form until ion exchange separation and metallographic reduction techniques were developed by Spedding and associates in 1950.
Physical
Appearance:  Dysprosium is bright silver in
color. As a salt it is typically greenish-yellow. It is relatively stable at room temperature but tarnishes in
moist air. It is soft enough to be cut with a knife.
Dysprosium is bright silver in
color. As a salt it is typically greenish-yellow. It is relatively stable at room temperature but tarnishes in
moist air. It is soft enough to be cut with a knife.

Dysprosium has 12 known isotopes ranging from Dy-154 to Dy-166
|
Isotope |
Half Life |
|
Dy-154 |
3000000.0 years |
|
Dy-156 |
Stable |
|
Dy-157 |
8.1 hours |
|
Dy-158 |
Stable |
|
Dy-159 |
144.4 days |
|
Dy-160 |
Stable |
|
Dy-161 |
Stable |
|
Dy-162 |
Stable |
|
Dy-163 |
Stable |
|
Dy-164 |
Stable |
|
Dy-165 |
2.3 hours |
|
Dy-166 |
3.4 days |
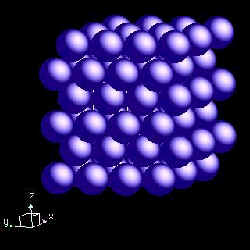
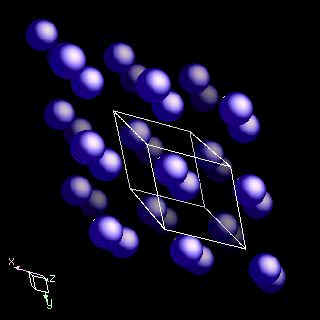
Dysprosium is hexagonal closest packed. The following diagrams illustrate this structure.
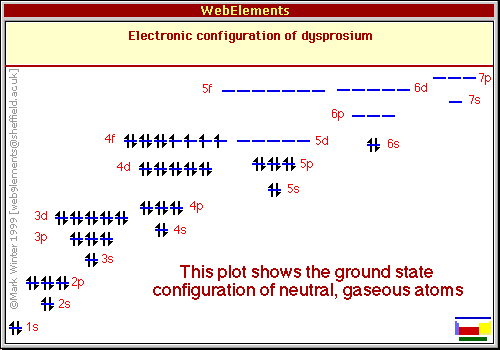
Ground State Electron Configuration:
[Xe]6s²4fış
Schematic Representation of shell structure-

Sources: Dysprosium occurs, along with other lanthanide series elements, in minerals such as xenotime, fergusonite, and gadolinite. Its most significant sources, however, are monaziate and bastnasite. It is difficult to separate as a metal, but can be extracted as a salt from ores with use of sulphuric acid, hydrocholric acid, and sodium hydroxide. Dysprosium can also be isolated through the reduction of dysprosium triflouride with calcium metal.
Uses: Currently there are not many applications for dysprosium. However, its unique properties perfectly suit it for particular uses. Its high melting point, and ability to absorb neutrons encourage its use in nuclear control applications. A dysprosium oxide cement is used to cool nuclear reactors.
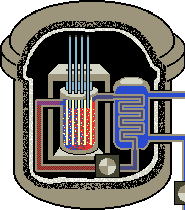
Dysprosium is used in the construction of color television tubes.

In combination with vanadium and other rare earth elements, dysprosium has been used to make lasers. Dysprosium-cadmium chalcogenides provide infrared radiation, and have been used to study chemical reactions.

Cost: Fortunately, the cost of dysprosium metal has dropped in recent years. Now the metal costs only about $300/ kg in purities of over 99%. This amounts to only 30 cents per gram!

Far Reaching Effects of Dysprosium: Dysprosium has had a profound effect on many people. It has been widely studied and scientists are searching for new applications. However, this effect is perhaps best shown by the Australian rock group who named their band after this fantastic element.
visit a related dysprosium site