Cost of 1 Kg of Extra Pure Iron Made By Reduction: $53.40
|
Cost of 1 Kg of Extra Pure Iron Made By Reduction: $53.40 |
Electronic data |
Thermal data |
Steric data |
||
|---|---|---|---|---|
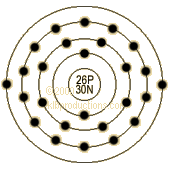
| Shells:
2,8,14,2 Orbitals: [Ar] 3d6 4s2 Electronegativity: 1.6, 1.8 1. Ionization potential: 7.9024 eV 2. Ionization potential: 16.18 eV 3. Ionization potential: 30.651 eV Oxidation states: 6,3,2,0,-2 Electrical conductivity: 0.0993 10^6 |
Melting
point: 1535 °C Boiling point: 2861 °C Specific heat: 0.44 J/gK Heat of fusion: 13.80 kJ/mol Heat of vaporization: 349.60 kJ/mol Thermal conductivity: 0.802 W/cmK |
Atomic
radius: 1.72 Å Ionic radius: 0.55 Å () Covalent radius: 1.17 Å Atomic volume: 7.1 cm³/mol Density (293 K): 7.86 g/cm³ Crystal structure: Cubic: Body centered
|
| Nuclide | Abundance [%] | Mass | Spin | Halflife | Decay mode | |
| 52Fe | 0 | 52 | 0 | 8.28h | ||
| 54Fe | 5.82 | 53.9396 | 0 | -- | stable | |
| 55Fe | 0 | 54.938 | 3/2 | 2.73y | EC | |
| 56Fe | 91.66 | 55.9349 | 0 | -- | stable | |
| 57Fe | 2.19 | 56.9354 | 1/2 | -- | stable | |
| 58Fe | 0.33 | 57.9333 | 0 | -- | stable | |
| 59Fe | 0 | 58.935 | 3/2 | 45.51d | ||
| 60Fe | 0 | 60 | 0 | 1.5E06y |
#Return to the top of the page
| Name origin: | Anglo-Saxon: iron; symbol from Latin: ferrum (iron) |
| Description: | Malleable, ductile, silvery-white metal. Fourth most abundant element in the earth's crust (56,300 ppm). Ninth most abundant element in the universe. |
| Discovered by: | Known to the ancients |
| Year: | -- |
| Place: | Unknown |
| Sources: | Obtained from iron ores. Pure metal produced in blast furnaces by layering limestone, coke and iron ore and forcing hot gasses into the bottom. This heats the coke red hot and the iron is reduced from its oxides and liquified where it flows to t |
| Use(s): | Used in steel and other alloys. Essential for humans. It is the chief constituent of hemoglobin which carries oxygen in blood vessels. Its oxides are used in magnetic tapes and disks. |
#Return to the top of the page
|
The use of Iron is PreHistoric. Genesis mentions Tubal-Cain, 7 generations descendant of Adam, was an "instructor of every artificer in brass and iron." |
|
|
In AD 400, a tremendous iron pillar was constructed in Dehli, India that still stands today. The pillar is one solid shaft of wrought iron 7 1/4 meters high and 40 cm in diameter. Corrosion was minimal though exposed to weather. |
|
Iron is very abundant in the universe, found in the sun and many types of stars in considerable quantity. |
|
Iron is characterized by a stable nuclei. |
|
Iron is the principle component of a class of meteorites known as 'siderites' and a minor constituent of two other classes. |
|
The core of the earth, 2150 m in radius, is believed to be largely composed of iron. |
|
4th most abundant element, by weight, composing the crust of the earth. |
|
Most common iron ore is hematite (Fe2O3). The metal is obtained by means of a reduction reaction with carbon. |
|
Iron is found in widely distributed minerals such as magnetite, a black sand found on beaches and shores. |
|
Common iron is a mixture of four isotopes. Six isotopes are known to exist. |
|
Iron is a vital component of plant and animal life, appearing, for instance, in hemoglobin. |
|
Pure metal is rarely encountered in commerce; Iron is usually alloyed with carbon or other alloys for use as in structural components amongst a multitude of other applications and uses. |
|
Iron is very reactive chemically, corroding in the presence of moisture or air. |
|
Pig Iron is composed of Iron and 3% carbon and varying amounts of S, Si, Mn, and P. Pig Iron is hard, brittle, fairly fusible, and used to produce other alloys such as steel. |
|
Wrought Iron contains a few tenths of a percent of carbon. Wrought iron is tough, malleable, less fusible, and is characterized by a fibrous structure. |
|
Carbon Steel is an alloy of carbon and iron, containing also small amounts of Mn, S, P, and Si. Alloy Steels are Carbon Steels with other additives such as nickel, chromium, vanadium, etc. |
Practical:
| American Iron and Steel Institute: http://www.steel.org/ | |
| Iron and Steel Society: http://www.issource.org/ | |
| Association of Iron and Steel Workers: http://www.aise.org/ |
Edible
| Iron Chef TV Show: http://www.foodtv.com/tvshows/ironchefindex/0,2243,,00.html | |
| Iron Chef Fan Club: http://www.ironchef.com/ |
Musical
| Iron Maiden: http://www.ironmaiden.com/ | |
| Iron Butterfly: http://www.ironbutterfly.com/ |
Entertainment
| Warner Brother's Iron Giant: http://irongiant.warnerbros.com/ | |
| Iron Butt Association: Long Distance Motorcycling: http://www.ironbutt.com/ |
#Return to the top of the page
| CRC Handbook of Chemistry and Physics. Editor-in-Chief: David R. Lyde, Ph. D. 1990-91. CRC Press: Boston. CopyRight 1974-90 by CRC Press. |
#Return to the top of the page
Comments? Concerns?